CEB-01 is a biocompatible and biodegradable membrane that locally and safely releases high doses of chemotherapy after the excision of a cancerous tumour.
The product meets an unmet oncological and surgical need, reducing the recurrence and spreading of tumoral cells and improving the local control of cancer after an intervention.
We have developed our own innovative proprietary technology, which has been patented in the United States, the European Union and China.

At Cebiotex we have created a new medical product through our proprietary technology in nanofiber engineering, alongside the medical and scientific teams of Sant Joan de Déu Barcelona Children’s Hospital (HSJD) and of the Polytechnic University of Catalonia (UPC).
The first candidate developed through our technological platform is CEB-01. It is a biocompatible and biodegradable nanofiber membrane loaded with the chemotherapeutic drug SN-38, indicated for the local control of surgical sites after an oncological intervention.
After the excision of a tumour, CEB-01 is placed on the surgical site and it immediately starts releasing SN-38 locally during weeks, until it biodegrades and disappears.
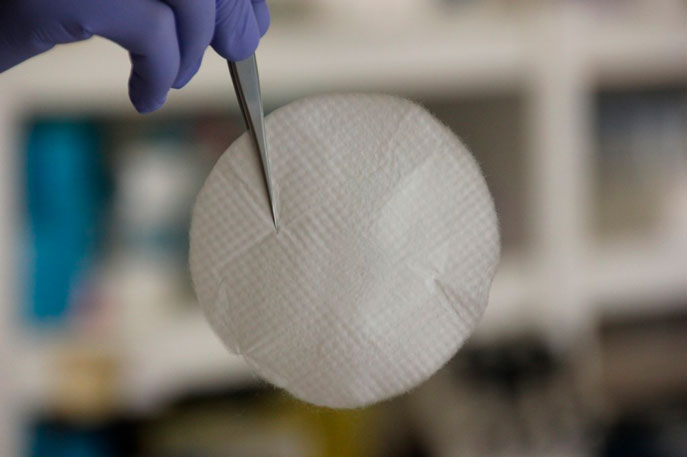
There is currently an unmet need in oncology and surgery: ensuring that the surgical site remains free of cancerous cells after a tumour excision. Compatible with the current Standard of Care, CEB-01 aims to solve such need by offering the option of intense local treatment during the time period in which no other therapy can be administered (up to 4 weeks after surgery.
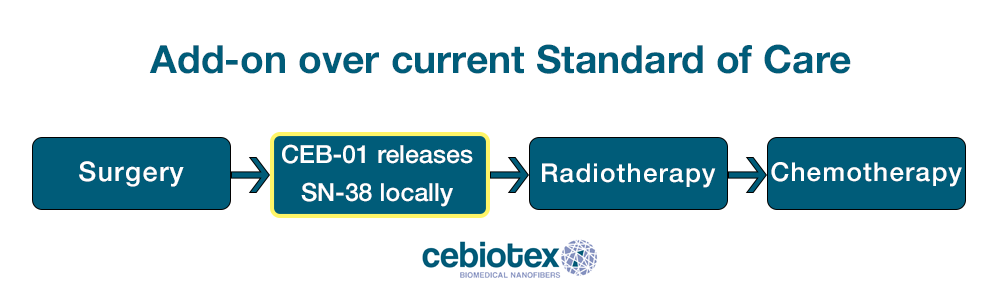
CEB-01 is an innovative solution that promises advantages in comparison to the conventional radiotherapy and chemotherapy application. It reduces the recurrence and the spreading of tumoral cells, improving the local control of cancer after an intervention.
“With this new therapeutic focus, we aim to reduce the secondary effects caused by chemotherapy, and especially radiotherapy in child cancer.”
Lucas Krauel, MD, PhD and CSO of Cebiotex
Our proprietary technology is protected under a patent in the United States, the European Union and China. Through this technology, we load the nanofiber membrane with a strong chemotherapy drug (SN-38) without chemical interaction between the polymer nanofibers and the applied agent.
In the future, we foresee the use of our technology applied not only in the oncology area but also in infections and advanced therapies, due to the fact that the material is biocompatible with other active ingredients.
“We have started with an orphan disease such as the sarcoma and we will continue on the paediatric line, but we are convinced that CEB-01 will become a standard after all oncological surgeries to ensure that the surgical site remains free of cancerous cells.”
Joan Bertran, CEO y cofundador de Cebiotex
