Uniquely reimagining the future together
What makes CEB-01 unique?
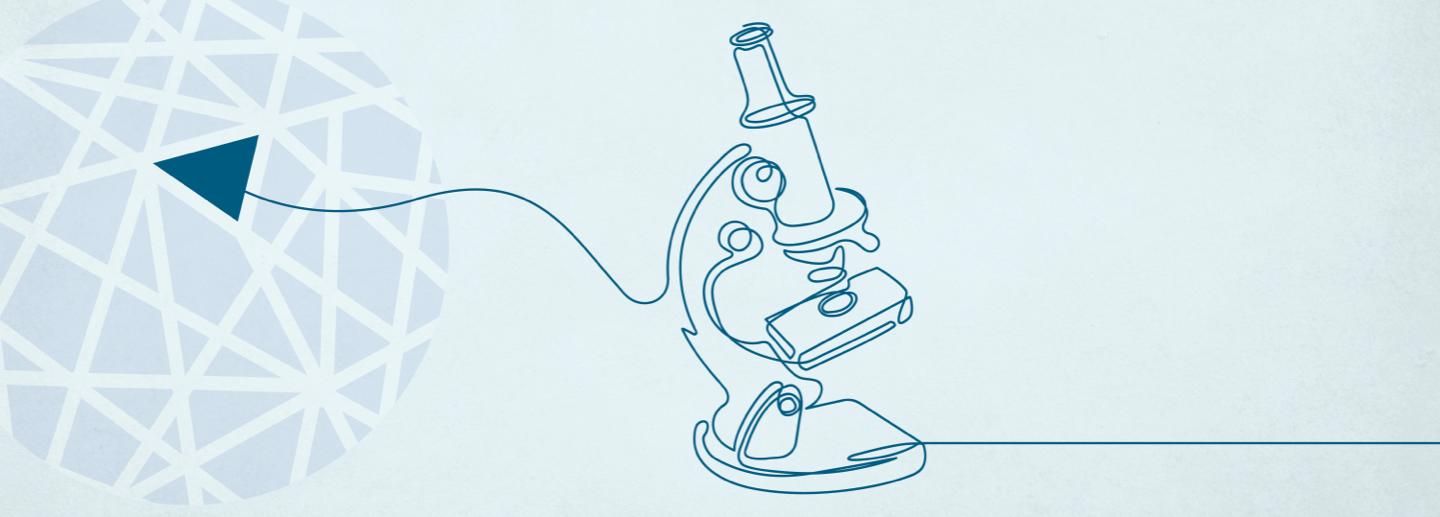
CEB-01 is the first local control system for soft tissue sarcoma (STS) whose safety, tolerability and efficacy have been demonstrated and validated in regulatory preclinical studies.
In addition, initial results from Phase I clinical development show that patients presented no toxicity or postoperative complications associated with CEB-01.
Lastly, we have our own GMP-graded machine for production, and both the production process and final product are GMP-certified.
There is no other product like CEB-01 on the market.
CEB-01 Phase I Clinical Trial
First clinical trial in humans (3+3, extension to 21 patients), to determine recommended doses and the safety and tolerance of PLGA CEB-01 membranes in patients with recurrent or locally advanced retroperitoneal STS after surgery.
- 5 active hospitals
- 12 patients: 4 women and 8 men
- Mean age: 62.1 years (range: 40–77)
Tumour:
- Dedifferentiated liposarcoma: 5
- Well-differentiated liposarcoma: 4
- Liposarcoma: 2
- Leiomyosarcoma: 1
5 are recurrences and 7 are new cases.
Conclusions
CEB-01 Phase I Clinical Trial
- CEB-01 is safe and well tolerated.
- DLT has not been observed with either dose.
- Adverse effects are similar to those following sarcoma surgery.
- Recurrence in the membrane area in 2 cases treated with the lowest dose.
- 5 patients with 1-2 years without local recurrence.